
crescentus, despite being only a few microns long, divides slightly off center to produce cells of different size and fates. Two newly described ectosymbionts hailing from the sulfur-oxidizing γ-proteobacteria divide using only one Z ring at the center of these long cells 146. This mechanism can be literally stretched to cells up to 120 microns in length.
coli grow by increasing their length and divide by binary fission to produce roughly equal-sized daughter cells, which are a few microns long. Inactivation of cytokinesis in organisms using such atypical modes of division often gives rise to diverse cell morphologies that are more complex than simple extension of a cylindrical rod.īox 1 – Variations on the theme of binary fission
#Binary fission in bacteria full
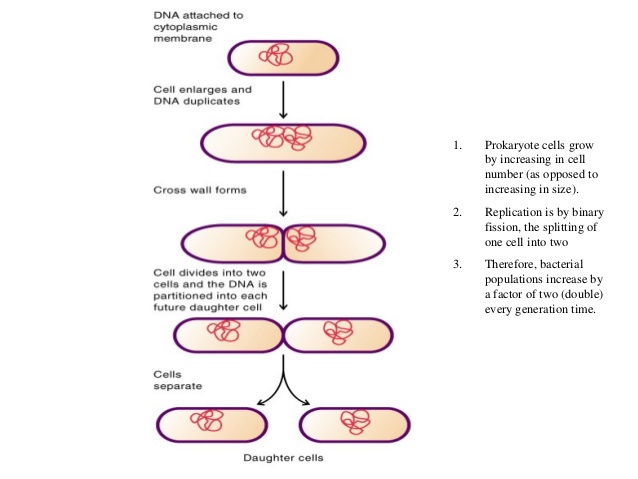
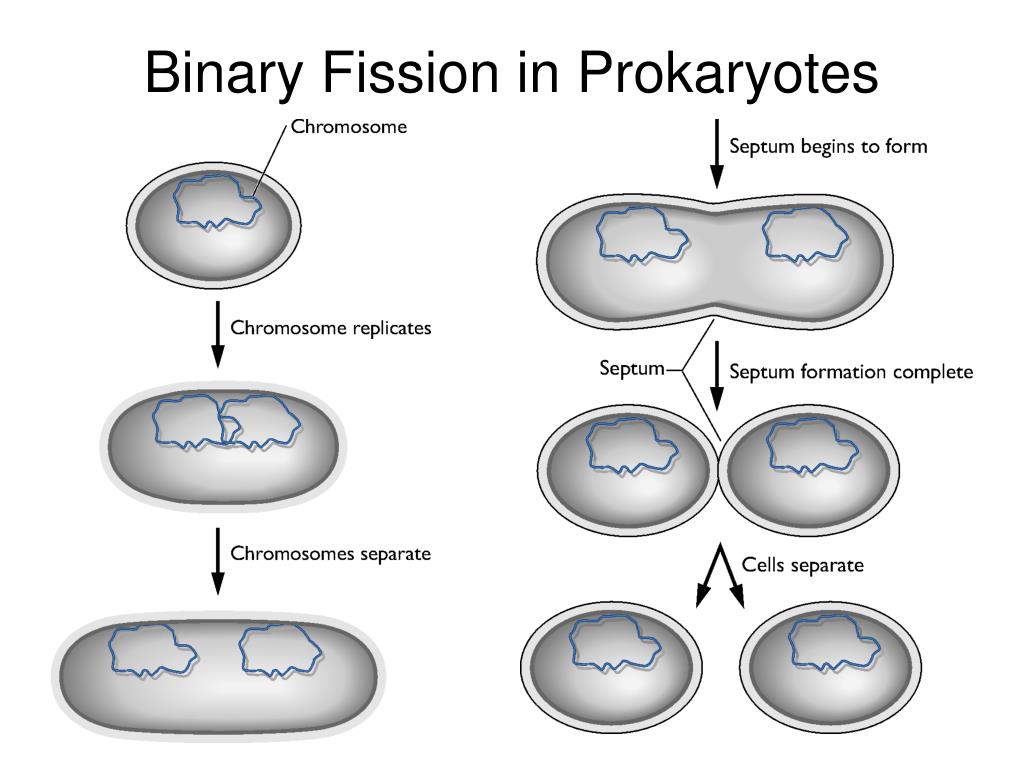
Finally, alternatives to binary fission such as polar budding 2, division without FtsZ 3, longitudinal division 4, and other non-canonical mechanisms showcase the vast diversity of bacterial strategies for cell duplication that still await full mechanistic explanations ( Box 1). Newly available genetic and cytological tools have allowed the study of cytokinesis in additional genera such as streptococci and mycobacteria that are rapidly emerging as additional model systems. When cytokinesis is inactivated in these and similar systems, cells continue to grow as cylindrical rods and chromosomes continue to replicate and segregate, resulting in filamentous cells with multiple nucleoids. coli and Caulobacter crescentus, as well as the Gram-positive Bacillus subtilis. Model bacteria that divide by binary fission include the Gram-negative E. For simplicity, many of the divisome proteins are not shown here, but they are shown in Fig. After septal peptidoglycan synthesis is underway, the other divisome proteins including the FtsBLQ complex transduce signals from the periplasm to the Z ring via FtsA, coordinating Z ring constriction (and FtsZ turnover) with septal peptidoglycan synthesis and targeted cell wall hydrolysis. FtsN’s periplasmic domain is targeted to peptidoglycan, reinforcing its localization and potentially stimulating FtsI and septal peptidoglycan synthesis. In addition, FtsA recruits FtsN via its cytoplasmic domain, which in turn may disrupt FtsA oligomers. In a later stage, ZipA bundling of FtsZ protofilaments is offset by FtsA-mediated disassembly. ZipA and FtsA are depicted as clustered, but they may be interspersed instead. (B) ZipA and FtsA tether FtsZ protofilaments and polymer bundles to the membrane using flexible linkers and either a transmembrane segment (ZipA, red rectangle) or an amphipathic helix (FtsA). The ultimate result is separation into two equal-sized daughter cells. After recruitment of additional proteins, the proto-ring progresses into a mature divisome, which coordinates constriction of the inner and outer membranes with targeted cell wall hydrolases and ingrowing septal peptidoglycan. coli cells replicate and segregate their chromosomes, organized as nucleoids, FtsZ and its membrane tethers are concentrated at midcell and organize into a Z ring or proto-ring. The divisome then constricts the cytoplasmic membrane, which in concert with synthesis of new peptidoglycan at the division septum and invagination of the outer membrane (in Gram-negative bacteria), achieves cytokinesis and cell separation. coli, and proteins that likely coordinate signaling between the these enzymes and the proto-ring such as FtsN and FtsBQL, thereby forming the mature divisome. In the second stage, the proto-ring recruits enzymes involved in cell wall (septum) synthesis such as FtsI in E. Membrane-associated FtsZ-interacting proteins such as FtsA and ZipA in Escherichia coli tether the Z ring to the cell envelope, resulting in an initial complex called the proto-ring ( Figure 1B, top). Initially, FtsZ undergoes GTP-dependent polymerization into filaments that form a defined ring-like structure (Z ring) at the site of cytokinesis. In several model bacterial species, the divisome has been proposed to assemble in temporally distinct stages ( Figure 1A). Fission is orchestrated by the divisome 1, a protein complex whose assembly is directed by the conserved tubulin homolog FtsZ. Most bacterial cells divide by binary fission, which is the splitting of a cell into two roughly equal progeny cells.


 0 kommentar(er)
0 kommentar(er)
